| CHE471: Chemical process principles I |
QUESTION 1
The rate of heat loss from a tank to the surrounding by convection and conduction is expressed by:
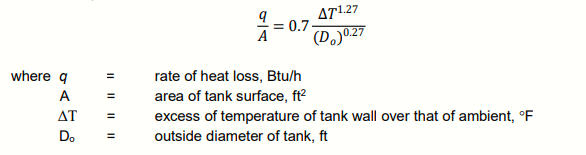
Determine the unit of constant 0.7. If the term q/A is expressed in J/minm2, ΔT inC, and Doin m, determine the new constant value.
QUESTION 2
Pure ethanol is also known as absolute alcohol. To qualify as “absolute,” the ethanol must contain no more than one percent water, or in other words, absolute alcohol is liquid alcohol that is at least 99% pure alcohol by weight. Pure ethanol can be produced from industrial alcohol via a process known as azeotropic distillation where a third component is introduced such as benzene.
The industrial alcohol is fed to an intermediate level of a distillation column. Benzene-rich stream is also fed to the column, commonly known as ‘reflux’. The vapor stream leaves the top of the column is a three-component mixture.
consisting of benzene, ethanol, and water while the liquid product leaves the bottom columnas pure ethanol. The overhead vapor product passes through a condenser and is completely condensed to a liquid. However, this liquid is actually having two separate immiscible phases, which are then separated in a decanter into an upper layer (benzene-rich)where this stream is then mixed with pure benzene as make-up for losses. This mixture is fed to the top of the column, as above mentioned. The lower layer, rich in ethanol and water is sent to another unit for benzene recovery. Table 1 shows the component weight analysis for each stream.
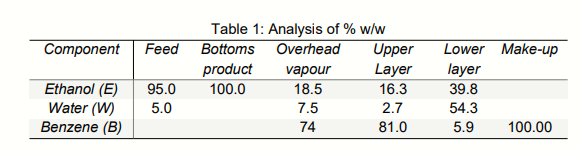
With the aid of a completely-labeled flowchart, for a feed rate to the process of 1200 kg/h, determine the production rate of absolute alcohol (kg/h), percentage recovery of ethanol from the feed as pure alcohol, flow rate (kg/h) of make-up benzene (pure benzene), and flowrates of upper and lower layers in the decanter (kg/h).
QUESTION 3
There are some electroplating factories around the Klang Valley area, that do not have good facilities for wastewater treatment. Wastewater from the electroplating process contains cyanide and metallic ions like copper, nickel, chromium, lead, zinc, silver, and others which are discharged directly into drains. Department of Environment (DOE) is the regulatory body for wastewater effluent quality through the Environmental Quality Act 1974 and its regulations such as the Environmental Quality (Sewage) Regulations 2009 and Environmental Quality(Industrial Effluent) Regulations 2009. The quality of surface water is determined by the water Quality Index and the suitability of surface water for irrigation is based on the designated classifications in the National Water Quality Standards for Malaysia established by DOE.
A silver (Ag) electroplating plant producing a wastewater stream contains 5 wt% Ag which needs to be treated before disposal. The wastewater is fed to a treatment tour where 95% of fed Ag is recovered, while residual liquid water is sent to a waste pond. The maximum capacity of the treatment tour is 6000 kg wastewater/hr. When the fed wastewater stream at a rate higher than the capacity of the treatment tour, the excess wastewater bypasses the unit and combines with the residual liquid water from the treatment tour and goes to the waste pond.

